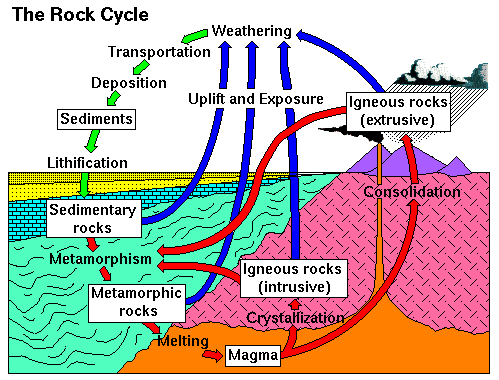
The solid Earth (the mantle and crust) is made of rock. You may have noticed that there are many kinds of rocks, from the soft sandy rocks that form the cliffs at Scripps beach to the hard rocks that form the mountains to the East of San Diego. Geologists have developed a way of classifying the various rocks and understand fairly well where they come from and where they go. There are three general types of rocks, those that form from melt (igneous rocks), those that are deposited from air or water (sedimentary rocks), and those that have formed by "cooking" or otherwise altering another rock (metamorphic rocks). Sedimentary rocks form by breaking down other kinds of rocks into small particles and washing or blowing them away; metamorphic rocks form from other rocks and igneous rocks form by melting other rocks. Thus rocks are always changing form and are redistributted as part of a giant cycle of renewal. This cycle is called the Rock Cycle.

(image copied from here)
Extrusive igneous rocks can be in the form of:
Intrusive rocks can be:
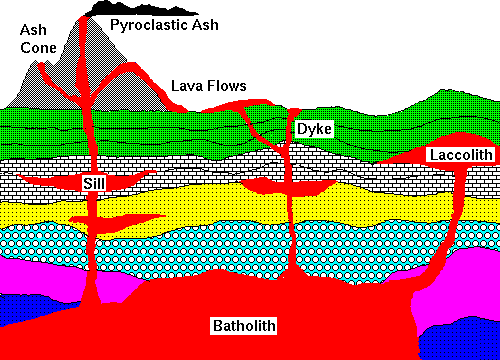
image copied from here
Sedimentary rocks: Rocks that are produced by the action of weathering and erosion that break down pre-existing rocks by physical and chemical processes. Sediment is the stuff that is transported by wind, water or ice to a site of deposition. There are three types of sediments:
Metamorphic rocks:
More than two hundred years ago, James Hutton lived and tramped the hills of Scotland. He was a keen observer and noticed many things about the world around him. He realized that some landforms can be created very quickly (i.e. the affect of floods, landslides, avalanches, volcanic eruptions), while others must take a long time (the building up and tearing down of mountains).
Hutton few quantitative ways to estimate how long geological features took to form. In order to estimate geological rates, Hutton developed the Principle of Uniformitarianism which states that, ``The present is the key to the past''). By watching what was going on around him, he guessed how geological formations must have been created, and how long they took to form. In this way, he realized that the Earth was quite old, at least a few million years old.
There are few ways It is possible to count tree rings, or annual layers in lakes.
The method of providing absolute ages to the geologic time scale became possible when radioactivity was discovered at the end of the last century. In the first few lectures I mentioned that certain isotopes of certain elements were unstable and underwent radioactive decay. Think about a pan of pop corn on the stove. Each kernel has the potential to pop, but they do it one at a time. You never know which particular kernel is going to pop, but you know if you wait long enough, most of them will have popped. All else being equal (i.e. temperature), the number of kernels popping at a given time is related to the number of kernels left in the pan. As the number of kernels dwindles, the number of popped corn kernels increases. You could plot the number of kernels left to be popped and the number that have already popped :
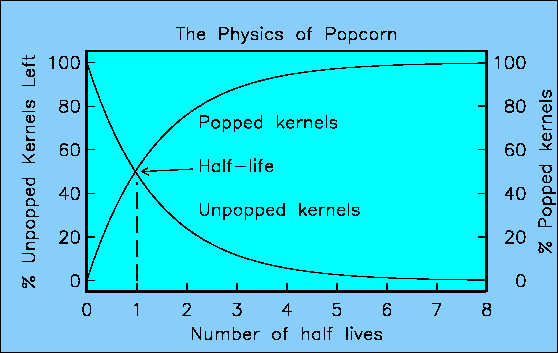
The time scale is determined by the half-life or the time it takes for half the kernels to pop. From looking at the graph, it is obvious that the number remaining at any time decreases with time, and more pop per unit time than pop later, when there are fewer left to pop. In fact, the number that pop during any interval depends on the number of unpopped kernels that were there at the beginning of the interval.
Mathematically, we can write this as:
The number of kernels remaining = [the number at the start] x [a special number] to the power of [(- time on stove) x (a time constant)]
The special number is called "e" and is about 2.71828. The time constant is some number that depends on the rate at which the pop corn pops. I used the number the natural log of 2 (0.6931), because then after one time unit, there are half the initial kernels left. You can actually plot the curve shown above with a hand calculator.
Try it:
Radioactivity behaves somewhat like popcorn as describe above. There are unstable isotopes of certain elements. These "parent" elements break down into other "child" elements by shedding particles from the nucleus. The rate at which this occurs depends only on the number of atoms around, so it follows exactly the same function as that described above. We can plot the numbers of parent and daughter atoms as we did for unpopped and popped kernels of popcorn:
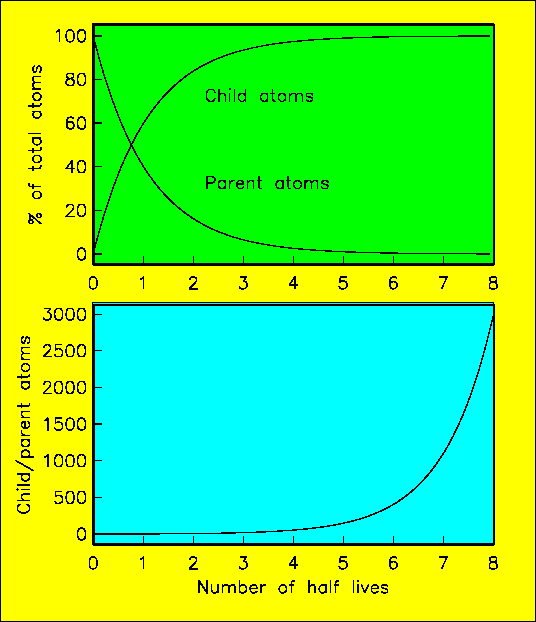
The decay constant of a particular parent can be measured in the laboratory by counting the number of times particles decay per second. If the decay constant of the parent is known, the age of a particular rock sample can be determined by comparing the ratio of parent to child, assuming there was no child in the sample to begin with, and none has been lost in the mean time. Decay rate is related to the half-life as you saw above. In fact, the half life = 0.6931/decay constant. (0.6931 is the natural logarithm of 2).
All radioactive elements decay in the same way, just some take a long time and some decay very rapidly. For a material to be useful to geologists, it has to have a half-life on the order of geologic processes and be around. Here is a list of commonly used isotopes and their half-lives:
Radioactive Parent |
Stable Daughter |
Half life |
|---|---|---|
Potassium 40 |
Argon 40 |
1.25 billion yrs |
Rubidium 87 |
Strontium 87 |
48.8 billion yrs |
Thorium 232 |
Lead 208 |
14 billion years |
Uranium 235 |
Lead 207 |
704 million years |
Uranium 238 |
Lead 206 |
4.47 billion years |
Carbon 14 |
Nitrogen 14 |
5730 years |
Because of the requirement that no child product be incorporated in the material to begin with, the minerals that are favored separate parent from child. Igneous rocks do this pretty well by excluding gases like argon and separating rubidium from strontium (these partition into different minerals during crystallization). Dating minerals in sediments generally will give you the age when the mineral formed - not the sedimentary rock, so geologists favor igneous rocks for dating purposes.
Most of the isotopes used for dating were made billions of years ago in a super-nova explosion, like the rest of the stuff we are made of. However, please observe the short half-life of for example, carbon-14. This could not possibly have survived from before the birth of the Earth and in fact is made in the upper atmosphere by bombardment of cosmic rays. Carbon is also different in that it is incorporated into organic material. It is used for dating things like trees, fires, cloth, soils, corals, etc. and is only good for the last 50,000 years or so.
Over the last few decades, geologists have found datable material in lots of useful places. We now know pretty well how long various parts of the rock cycle take. For example, we know that it takes millions of years to build a mountain and hundreds of millions of years to wear it back down. It takes tens of thousands of years to make a decent soil and only a few years to wash it away. We can now estimate how rapidly can climate change (Frighteningly fast!).